|
Chemistry: Methods and Measurement
-
State the
definition of chemistry and discuss its interrelationship with
other fields of science and medicine.
-
Describe the
approach to science, the scientific method.
-
Distinguish
among the terms hypothesis, theory and scientific law.
-
State both
the differences and relationships between science and
technology.
-
Distinguish
between data and results.
-
Learn the
major units of measure in the English and metric systems, and be
able to convert from one system to another.
-
Compare and
contrast the terms error, accuracy, precision, and
uncertainty
-
Report data
and results using scientific notation and the proper number of
significant figures.
-
Use
appropriate experimental quantities in problem solving.
-
Calculate the
density of an object from mass and volume data and calculate the
specific gravity of an object from its density.
The Composition and Structure of the Atom
-
Describe the
properties of the solid, liquid, and gaseous state.
-
Classify
properties as chemical or physical.
-
Classify
observed changes in matter as chemical or physical.
-
Provide
specific examples of physical and chemical properties.
-
Distinguish
between intensive and extensive properties.
-
Classify
matter as element, compound, or mixture.
-
Recognize the
interrelationship of the structure of matter and its physical
and chemical properties.
-
Describe the
important properties of protons, neutrons, and electrons.
-
Calculate the
number of protons, neutrons, and electrons in any atom.
-
Distinguish
among atoms, ions, and isotopes.
-
Trace the
history of development of atomic theory, beginning with Dalton.
-
Summarize the
experimental basis for the discovery of the charged particles
and the nucleus.
-
Explain the
critical role of spectroscopy in the development of atomic
theory and in our everyday lives.
-
State the
basic postulates of Bohr’s theory.
-
Compare and
contrast Bohr’s theory and the more sophisticated
“wave-mechanical” approach.
Elements, Atoms, Ions, and the Periodic Table
-
Recognize the
important subdivisions of the periodic table:
periods, groups (families), metals, and nonmetals
-
Use the
periodic table to obtain information about an element.
-
Describe the
relationship between the electronic structure of an element and
its position in the periodic table.
-
Write
electron configurations for atoms of the most commonly occurring
elements.
-
Know the
meaning of the octet rule and its predictive usefulness.
-
Use the octet
rule to predict the charge of common cations and anions.
-
Utilize the
periodic table and its predictive power to estimate the relative
sizes of atoms and ions, as well as relative magnitudes of
ionization energy and electron affinity.
-
Use values of
ionization energies and electron affinities to predict ion
formation.
Structure and Properties of Ionic and Covalent
Compounds
-
Classify
compounds as having ionic, covalent, or polar covalent bonds.
-
Name common
inorganic compounds using standard conventions and recognize the
common names of frequently used substances.
-
Write the
formulas of compounds when provided with the name of the
compound.
-
Predict the
differences in physical state, melting and boiling points,
solid-state structure, and solution chemistry that result from
differences in bonding.
-
Draw Lewis
structures for covalent compounds and complex inorganic ions.
-
Describe the
relationship between stability and bond energy.
-
Predict the
geometry of molecules and ions using the octet rule and Lewis
structure.
-
Understand
the role that molecular geometry plays in determining the
solubility and melting and boiling points of compounds.
-
Use the
principles of VSEPR theory and molecular geometry to predict
relative melting points, boiling points, and solubilities of
compounds.
Calculations and the Chemical Equation
-
Know the
relationship between the mole and Avagadro’s number, and the
usefulness of these quantities.
-
Perform
calculations using Avagadro’s number and the mole.
-
Write
chemical formulas for common inorganic substances.
-
Calculate the
formula weight and molar mass of a compound.
-
Know the
major function served by the chemical equation, the basis for
chemical calculations.
-
Balance
chemical equations given the identity of products and reactants.
-
Calculate the
number of moles of product resulting from a given number of
moles of reactants or the number of moles of reactant needed to
produce a certain number of moles of product.
-
Perform
calculations involving limiting reactants to determine the
theoretical yield of a reaction.
-
Calculate
theoretical and percent yield.
Reactions and Solutions
-
Classify
chemical reactions by type: combination
(synthesis), decomposition, or replacement (single or double).
-
Recognize the
various classes of chemical reactions:
precipitation, reactions with oxygen, acid-base, and
oxidation-reduction
-
Distinguish
among the terms solution, solute, and solvent.
-
Describe
various kinds of solutions, and give examples of each.
-
Describe the
relationship between solubility and equilibrium.
-
Calculate
solution concentration in weight/volume percent and
weight/weight percent.
-
Calculate
solution concentration using molarity.
-
Perform
dilution calculations.
-
Interconvert
molar concentration of ions and milliequivalents/liter.
-
Describe and
explain concentration-dependent solution properties.
-
Describe why
the chemical and physical properties of water make it a truly
unique solvent.
-
Explain the
role of electrolytes in blood and their relationship to the
process of dialysis.
States of Matter: Gases,
Liquids, and Solids
-
Describe the
behavior of gases expressed by the gas laws:
Boyle’s law, Charles’s law, combined gas law, Avagadro’s
law, the ideal gas law, and Dalton’s law.
-
Use gas law
equations to calculate conditions and changes in conditions of
gases.
-
Describe the
major points of the kinetic molecular theory of gases.
-
Explain the
relationship between the kinetic molecular theory and the
physical properties of macroscopic quantities of gases.
-
Describe
properties of the liquid state in terms of the properties of the
individual molecules that comprise the liquid.
-
Describe the
process of melting, boiling, evaporation, and condensation.
-
Describe the
dipolar attractions known collectively as van der Waals forces.
-
Describe
hydrogen bonding and its relationship to boiling and melting
temperatures.
-
Relate the
properties of the various classes of solids (ionic, covalent,
molecular, and metallic) to the structure of these solids.
Chemical and Physical Change:
Energy, Rate, and Equilibrium
-
Correlate the
terms endothermic and exothermic with heat flow
between a system and its surroundings.
-
State the
meaning of the terms enthalpy, entropy, and free
energy and know their implications.
-
Describe
experiments that yield thermochemical information and calculate
fuel value based on experimental data.
-
Describe the
concept of reaction rate and the role of kinetics in chemical
and physical change.
-
Describe the
importance of activation energy and the activated
complex in determining reaction rate.
-
Predict the
way reactant structure, concentration, temperature, and
catalysis affect the rate of a chemical reaction.
-
Write rate
equations for elementary processes.
-
Recognize and
describe equilibrium situations.
-
Write
equilibrium-constant expressions and use these expressions to
calculate equilibrium constants.
-
Use
LeChatelier’s principle to predict changes in equilibrium
position.
Charge Transfer Reactions:
Acids and Bases and Oxidation-Reduction
-
Identify
acids and bases and acid-base reactions.
-
Write
equations describing acid-base dissociation and label the
conjugate acid-base pairs.
-
Describe the
role of the solvent in acid-base reactions, and explain the
meaning of the term pH.
-
Calculate pH
from concentration data.
-
Calculate
hydronium and/or hydroxide ion concentration from pH data.
-
Provide
examples of the importance of pH in chemical and biochemical
systems.
-
Describe the
meaning and utility of neutralization reactions.
-
State the
meaning of the term buffer and describe the applications
of buffers to chemical and biochemical systems, particularly
blood chemistry.
-
Describe
oxidation and reduction, and describe some practical
examples of redox processes.
-
Diagram a
voltaic cell and describe its function.
-
Compare and
contrast voltaic and electrolytic cell.
The Nucleus, Radioactivity, and Nuclear Medicine
-
Enumerate the
characteristics of alpha, beta, and gamma radiation.
-
Write
balanced equations for common nuclear processes.
-
Calculate the
amount of radioactive substance remaining after a specified
number of half-lives.
-
Describe the
various ways in which nuclear energy many be used to generate
electricity: fission, fusion, and the
breeder reactor.
-
Explain the
process of radiocarbon dating.
-
Cite several
examples of the use of radioactive isotopes in medicine.
-
Describe the
use of ionizing radiation in cancer therapy.
-
Discuss the
preparation of radioisotopes for use in diagnostic imaging
studies.
-
Explain the
difference between natural and artificial radioactivity.
-
Describe the
characteristics of radioactive materials that relate to
radiation exposure and safety.
-
Be familiar
with common techniques for the detection of radioactivity.
-
Know the
common units in which radiation intensity is represented:
the curie, roentgen, rad, and rem.
An Introduction to Organic Chemistry:
The Saturated Hydrocarbons
-
Compare and
contrast organic and inorganic compounds.
-
Draw
structures that represent each of the families of organic
compounds.
-
Write the
names and draw the structures of the common functional groups.
-
Write
condensed and structural formulas for saturated hydrocarbons.
-
Describe the
relationship between the structure and physical properties of
saturated hydrocarbons.
-
Use the basic
rules of the IUPAC Nomenclature Sysytem to name alkanes and
substituted alkanes.
-
Draw
constitutional isomers of simple organic compounds.
-
Write the
names and draw the structures of simple cycloalkanes.
-
Draw cis
and trans isomers of cycloalkanes.
-
Describe
conformations of alkanes.
-
Draw the
chair and boat conformations of cyclohexane.
-
Write
equations for the combustion reactions of alkanes.
-
Write
equations for halogenation reactions of alkanes.
The Unsaturated Hydrocarbons:
Alkenes, Alkynes, and Aromatics
-
Describe the
physical properties of alkenes and alkynes.
-
Draw the
structures and write the IUPAC names for simple alkenes and
alkynes.
-
Write the
names and draw the structures of simple geometric isomers of
alkenes.
-
Write
equations predicting the products of addition reactions of
alkenes: hydrogenation, halogenation,
hydration, and hydrohalogenation.
-
Apply
Markovnikov’s rule to predict the major and minor products of
the hydration and hydrohalogenation reactions of unsymmetrical
alkenes.
-
Write
equations representing the oxidation of simple alkenes.
-
Write
equations representing the formation of addition polymers of
alkenes.
-
Draw the
structures and write the names of common aromatic hydrocarbons.
-
Write
equations for substitution reactions involving benzene.
-
Describe
heterocyclic aromatic compounds and list several biological
molecules in which they are found.
Learning Goals...with more specifics
Introduction:
Matter and Measurement
Matter: Elements,
Compounds, and Mixtures
- Distinguish between
physical and chemical properties and also between simple and
physical and chemical changes.
- Differentiate between the
three states of matter.
- Distinguish between
elements, compounds, and mixtures.
- Give the symbols for the
elements discussed in class.
Physical Quantities and
Units
- You should be able to list
the basic SI and metric units and the commonly used prefixes in
scientific measurement.
Uncertainty in
Measurements: Significant Figures
- Determine the number of
significant figures in a measured quantity.
- Express the result of a
calculation with the proper number of significant figures.
Temperature and Density:
Intensive Properties
- Convert temperatures among
the Fahrenheit, Celsius, and Kelvin scales.
- Perform calculations
involving density.
Dimensional Analysis
- You should be able to
convert between units by using dimensional analysis.
Atoms, Molecules, and
Ions
Atoms
- Describe the composition
of an atom in terms of protons, neutrons, and electrons.
- Give the approximate size,
relative mass, and charge of an atom, proton, neutron, and
electron.
- Write the chemical symbol
for an element, having been given its mass number and atomic
number and perform the reverse operation.
- Describe the properties of
the electron as seen in cathode rays. Describe the means by
which J.J. Thomson determined the ratio e/m for the
electron.
- Describe Millikan's
oil-drop experiment and indicate what property of the electron
he was able to measure.
- Cite the evidence from
studies of radioactivity for the existence of subatomic
particles.
- Describe the experimental
evidence for the nuclear nature of the atom.
Molecules and Ions:
Relationships in the Periodic Table
- Write the symbol and
charge for an atom or ion, having been given the number of
protons, neutrons, and electrons, and perform the reverse
operation.
- Use the periodic table to
predict the charges of monatomic ions.
- Use the periodic table to
predict whether an element is a metal or a nonmetal.
- Distinguish between
empirical formulas, molecular formulas and structural formulas.
Nomenclature
- Write the simplest formula
for a compound, having been given the charges of the ions from
which it is made.
- Write the name of a simple
inorganic compound, having been given its chemical formula, and
perform the reverse operation.
Stoichiometry:
Calculations with Chemical Formulas and Equations
Chemical Equations:
Balancing and Predicting Products of Reactions
- Balance chemical
equations.
- Predict the products of a
chemical reaction, having seen a suitable analogy.
- Predict the products of
the combustion reactions of hydrocarbons and simple compounds
containing C, H, and O atoms.
Atomic Weight, Molecular
Weight, and the Mole
- Calculate the atomic
weight of an element given the abundances and masses of its
isotopes.
- Calculate the molecular
weight and molar mass of a substance from its chemical formula.
- Interconvert number of
moles and mass of a substance. Use Avogadro's number and molar
mass to calculate the number of particles making up a substance,
and vice versa.
Determination of Empirical
and Molecular Formulas
- Calculate the empirical
formula of a compound, having been given appropriate analytical
data such as elemental percentages or the quantity of CO2
and H2O produced by combustion.
- Calculate the molecular
formula, having been given the empirical formula and molecular
weight.
Chemical Equations:
Mass and Mole Relationships
- Calculate the mass of a
particular substance produced or used in a chemical reaction
(mass-mass problem).
- Determine the limiting
reagent in a reaction.
- Calculate the theoretical
and actual yields of chemical reactions given the appropriate
data.
Aqueous Reactions and
Solution Stoichiometry
Aqueous Solutions:
Electrolytes and Acids and Bases
- Predict whether a
substance is a nonelectrolyte, strong electrolyte, or weak
electrolyte from its chemical behavior.
- Predict the ions formed by
electrolytes when they dissociate of ionize.
- Identify substances as
acids, bases, or salts.
Precipitation Reactions:
Ionic Equations
- Use solubility rules to
predict whether a precipitate forms when electrolyte solutions
are mixed.
- Predict the products of
metathesis reactions (including both neutralization and
precipitation reactions) and write balanced chemical equations
for them.
- Identify the spectator
ions and write the net ionic equations for solution reactions,
starting with their molecular equations.
Oxidation and Reduction:
Oxidation Numbers and Activity Series
- Determine whether a
chemical reaction involves oxidation and reduction.
- Assign oxidation numbers
to atoms in molecules and ions.
- Use the activity series to
predict whether a reaction will occur when a metal is added to
an aqueous solution of either a metal salt of an acid; and write
the balanced molecular and net ionic equation for the reaction.
Concentration of Solutions
- Calculate molatiry;
solution volume, or number of moles of solute given any two of
these quantities.
- Calculate the volume of a
more concentrated solution that must be diluted to obtain a
given quantity of a more dilute solution.
Solution Stoichiometry
- Calculate the volume of a
solution required to react with a volume of a different solution
using molarity and the stoichiometry of the reaction.
- Calculate the amount of a
substance required to react with a given volume of a solution
using molarity and the stoichiometry of the reaction.
- Calculate the
concentration or mass of solute in a sample from titration data.
Thermochemistry
Thermodynamics: The
First Law and Internal Energy Changes
- Give examples of different
forms of energy.
- List the important units
in which energy is expressed and convert from one to another.
- Define the first law of
thermodynamics both verbally and by means of an equation.
- Describe how the change in
internal energy of a system is related to the exchange of heat
and work between the system and its surroundings.
- Define the term state
function and describe its importance in thermochemistry.
Enthalpy: Heats of
Reactions
- Define enthalpy, and
relate the enthalpy change in a process occurring at constant
pressure to the heat added to or lost by the system during the
process.
- Sketch an energy diagram,
given the enthalpy changes in the processes involved, and
associate the sign of DH
with whether the process is exothermic or endothermic.
- Calculate the quantity of
heat involved in a reaction at constant pressure given the
quantity of reactants and the enthalpy change for the reaction
on a mole basis.
Calorimetry: Fuel
Values
- Define the terms heat
capacity and specific heat.
- Calculate any one of the
following quantities given the other three: heat, quantity of
material, temperature change, and specific heat.
- Calculate the heat
capacity of a calorimeter, given the temperature change and
quantity of heat involved; also calculate the heat evolved or
absorbed in a process from a knowledge of the heat capacity of
the system and its temperature change.
- Define the term fuel
value; calculate the fuel value of a substance given its
heat of combustion or estimate the fuel value of a material
given its composition.
- List the major sources of
energy on which humankind must depend, and discuss the likely
availability of these for the foreseeable future.
Hess's Law
- State Hess's law, and
apply it to calculate the enthalpy change in a process, given
the enthalpy changes in other processes that could be combined
to yield the reaction of interest.
Heats of Formation:
Calculating Heats of Reactions
- Define and illustrate what
is meant by the term standard state, and identify the
standard states for the elements carbon, hydrogen, and oxygen.
- Define the term
standard heat of formation, and identify the type of
chemical reaction with which it is associated.
- Calculate the enthalpy
change in a reaction occurring at constant pressure, given the
standard enthalpies of formation of each reactant and product.
Electronic Structures
of Atoms
Electromagnetic Radiation
- Describe the wave
properties and characteristic speed of propagation of radiant
energy (electromagnetic radiation).
- Use the relationship
ln
= c, which relates the wavelength (ll)
and the frequency (n)
of radiant energy to its speed (c).
Quantization of Energy
- Explain the essential
feature of Planck's quantum theory, namely, the smallest
increment, or quantum, of radiant energy of frequency,
n, that can
be emitted or absorbed is hn,
where h is Planck's constant.
- Explain how Einstein
accounted for the photoelectric effect by considering the
radiant energy to be a stream of particle-like photons striking
a metal surface. In other words, you should be able to explain
all the observations about the photoelectric effect using
Einstein's model.
Line Spectra and the Bohr
Model
- Explain the origin of the
expression line spectra.
- List the assumptions made
by Bohr in his model of the hydrogen atom.
- Explain the concept of an
allowed energy state and how this concept is related to the
quantum theory.
- Calculate the energy
differences between any two allowed energy states of the
electron in hydrogen.
- Explain the concept of
ionization energy.
Principles of Modern
Quantum Theory
- Calculate the
characteristic wavelength of a particle from a knowledge of its
mass and velocity.
- Describe the uncertainty
principle and explain the limitation it places on our ability to
define simultaneously the location and momentum of a subatomic
particle, particularly an electron.
- Explain the concepts of
orbital, electron density, and probability as used in the
quantum-mechanical model of the atom. Explain the physical
significance of Y2.
- Describe the quantum
numbers, n, l, m, used to define an orbital in an atom
and list the limitations placed on the values each may have.
- Describe the shapes of the
s, p, and d orbitals.
Energies of Orbitals in
Many-Electron Atoms
- Explain why electrons
with the same value of principal quantum number (n) but
different values of the azimuthal quantum number (l)
possess different energies.
Electronic Structure of
Many-Electron Atoms
- Explain the concepts of
electron spin and the electron spin quantum number.
- State the Pauli exclusion
principle and Hund's rule, and illustrate how they are used in
writing the electronic structures of the elements.
- Write the electron
configuration for any element.
- Write the orbital diagram
representation for electron configurations of atoms.
The Periodic Table:
Periodic Arrangement of Electron Configurations and Valence Electrons
Electromagnetic Radiation
- Describe what we mean by
the s, p, d, and f blocks of elements.
- Write the electron
configuration and valence electron configuration for any element
once you know its place in the periodic table.
Periodic Properties
of the Elements
Atomic Properties:
Atomic Size, Ionization Energy and Electron Affinity
- Explain the effect of
increasing nuclear charge on the radial density function in
many-electron atoms.
- Explain the variations in
bonding atomic radii among the elements, and predict the
relative sizes of atoms based on their positions in the periodic
table.
- Explain the observed
changes in values of the successive ionization energies for a
given atom.
- Explain the general
variations in first ionization energies among the elements, and
relate these variations to variations in atomic radii.
- Explain the variations in
electron affinities among the elements.
Overview: Metals and
Nonmetals
- Describe the periodic
trends in metallic and nonmetallic behavior.
- Describe the general
differences in chemical reactivity between metals and nonmetals.
Group Trends Exemplified:
The Active Metals
- Describe the general
physical and chemical behavior of the alkali metals and
alkaline earth metals, and explain how their chemistry relates
to their position in the periodic table.
- Write balanced equations
for the reaction of hydrogen with metals to form metal
hydrides.
- Write balanced equations
for simple reactions between the active metals (groups 1A and
2A) and the nonmetals in groups 6A and 7A.
Group Trends Exemplified:
Selected Nonmetals
- Write balanced equations
for the reaction of hydrogen with non-metals such as oxygen and
chlorine.
- Describe the allotropy of
oxygen.
- Explain the dominant
chemical reactions of oxygen and relate this behavior to it
position in the periodic table.
- Describe the physical
states and colors of the halogens, and explain the trends in
reactivity with increasing atomic number in the family.
- Explain the very low
chemical reactivity of the noble gas elements.
Basic Concepts of
Chemical Bonding
Lewis Symbols: Octet
Rule
- Determine the number of
valence electrons for any atom, and write its Lewis symbol.
- Recognize when the octet
rule applies to the arrangement of electrons in the valence
shell for an atom.
Ionic Bonding:
Energy, Ions, and Ionic Size
- Describe the origin of the
energy terms that lead to stabilization of ionic lattices.
- Predict on the basis of
the periodic table the probable formulas of ionic substances
formed between common metals and nonmetals.
- Describe how the radii of
ions relates to those of atoms.
- Explain the concept of an
isoelectronic series and the origin of changes in ionic radius
within a series.
The Lewis Model for
Covalent Bonding
- Describe the basis of the
Lewis theory, and predict the valence of common nonmetallic
elements from their positions in the periodic table.
- Be able to describe a
covalent bond in terms of sharing of electron density between
bonded atoms.
- Describe the formation of
a covalent compound using Lewis symbols.
- Be able to look at a Lewis
structure and determine if it properly fits the Lewis model.
- Describe a single, double,
and triple covalent bond.
Bond Polarity,
Electronegativity, and Nomenclature
- Explain the significance
of electronegativity and in a general way relate the
electronegativity of an element to its position in the periodic
table.
- Predict the relative
polarities of bonds using either the periodic table or
electronegativity values.
- Name a binary compound
given its chemical formula or write the chemical formula given
its chemical name.
Drawing Lewis Structures
- Write the Lewis structures
for molecules and ions containing covalent bonds, using the
periodic table.
- Write resonance forms for
molecules or polyatomic ions that are not adequately described
by a single Lewis structure.
Exceptions to the Octet
Rule
- You should be able to
write the Lewis structures for molecules and ions containing
covalent bonds that have an odd number of electrons, a
deficiency of electrons, or an expanded octet.
Strengths of Covalent Bonds
- You should be able to
relate bond enthalpies to bond strengths and use bond enthalpies
to estimate DH
for reactions.
Molecular Geometry
and Bonding Theories
VSEPR Model: A Tool
for Predicting Molecular Structure and Dipole Moments
- Relate the number of
electron domains in the valence shell of an atom in a molecule
to the geometrical arrangement around the atom.
-
|
Curriculum Guide for NCHS Chemistry By Objective
|
|
Goals
|
Content
|
Honors/Advanced
|
| COMPETENCY GOAL 1: The learner will build an understanding of the structure and properties of matter. (30% of curriculum) |
1.01 Summarize the development of current atomic theory.
(2%) |
John Dalton's atomic theory.
J. J. Thomson - discovery of the electron
E. Rutherford gold foil experiment, nucleus
R. A. Millikan - charge on the electron
N. Bohr - Hydrogen spectrum and electron arrangement
(No names will be tested) |
|
1.02 Examine the nature of atomic structure:
1.021 Protons.
1.022 Neutrons.
1.023 Electrons.
1.023 Atomic mass.
1.024 Atomic number.
1.025 Electron configuration.
1.026 Energy levels.
1.027 Isotopes.
(4%) |
Properties of sub-atomic particles: relative mass, charge, and location in atom
Symbols: A and Z,
Principle quantum numbers; s, and p sublevels;
Electron configuraton
Orbitals notation using up/down arrows for opposite spin
Valence electrons
Lewis electron dot diagrams for atoms
Isotope notation: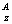 E (ie. E (ie.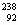 U or U-238) U or U-238)
Identify isotopes by mass and atomic number
|
Quantum numbers: azimuthal, magnetic and spin
Computation of energies and wavelengths in H spectrum.
|
1.03 Apply the language and symbols of chemistry
(4%) |
Binary nomenclature: Stock system for metal-nonmetal compounds and Greek prefix system for nonmetal-nonmetal compounds.
Stock system for compounds with polyatomic ions.
State symbols: (s), (l), (g)
Name the 6 strong acids and acetic.
Arrows indicating reactions and equilibria. |
Organic nomenclature, functional groups and named reactions. |
1.04 Identify substances using their physical properties:
1.041 Melting points.
1.042 Boiling points.
1.043 Density.
1.044 Color.
1.045 Solubility. (4%) |
Identify substances using their physical properties. Students should be able to read and apply information from the reference tables. |
|
| 1.05 Analyze and explain the nature and behavior of the atomic nucleus including radioactive isotopes and their practical application. (4%) |
Characteristics of alpha, beta, gamma radiation: Relative masses, charges, symbols, penetrating ability; Shielding: air (alpha), metal (beta), and distance (qualitative use of inverse square law). Concepts of half-life, fission, and fusion.
Uses: dating, cancer therapy, smoke detectors, imaging. |
Decay equations.
Inverse square law calculations. |
1.06 Analyze the basic assumptions of kinetic molecular theory and its applications:
1.061 Ideal Gas Equation.
1.062 Combined Gas Law.
1.063 Graham’s Law.
1.064 Dalton’s Law of Partial Pressures.
(4%) |
Five assumptions of KMT:
Avogadro's Law, PV=nRT, Boyle's Law, Charles' Law, P1V1/T1=P2V2/T2,
1 mole of any gas at STP = 22.4 L
Differentiate between real and ideal gases (factors nor calculations)
Graham's Law
Pt=P1+P2+ ... ; collecting a gas over water and vapor pressure of water. |
Calculations of KE or speeds of molecules, Maxwell's distribution
Calculate molecular weight from effusion of gases
|
1.07 Assess the structure of compounds relating bonding and molecular geometry to chemical and physical properties;
1.071 Ionic bonds.
1.072 Covalent bonds.
1.073 Metallic bonds.
(3%) |
Electronegativity general trend - predict nature of bond.
Ion formation and stable arrangements (i.e. inert gas structure)
prediction of physical properties based on bonding (melting point etc.)
Lewis structures including single, double, triple bonds
VSEPR Theory:
Geometry: linear, bent, trigonal planar and tetrahedral, trigonal pyramidal.
Polar / nonpolar bonds, polar / nonpolar molecules and solubility in polar or nonpolar solvents. ("like dissolves like"). Include intermolecular forces to explain polarity. |
Resonance.
Formal charge calculations.
Geometries: trigonal bipyramidal, octahedral.
Hybrid orbital theory.
Molecular orbital theory.
|
| COMPETENCY GOAL 2: The learner will build an understanding of regularities in chemistry. ( 36% of curriculum) |
2.01 Analyze periodic nature of trends in chemical properties and examine the use of the Periodic Table to predict properties of elements;
2.011 Symbols.
2.012 Groups(families).
2.013 Periods.
2.014 Transition elements.
2.015 Ionization energy.
2.016 Atomic and ionic radii.
2.017 Electronegativity
(5%) |
Define family (group) and period.
Location on PT of alkali metals, alkaline earth metals, transition metals, rare earth metals, metalloids, halogens, inert gases. Also s, p, and d block elements.
General trends of electronegativity, and ionization energy.
Use PT to predict chemical and physical properties as well as charge of ions.
General trends in atomic and ionic radii,
Relate periodicity to electron configurations.
Students will always have PT to use. |
|
2.02 Analyze the mole concept and Avogadro's number and use them to calculate:
2.021 Mole to molecule.
2.022 Mass to moles.
2.023 Volume of a gas to moles.
2.024 Solution concentrations.
(5%)
|
Conversion factors using moles, mole-mole, mole-mass, mass-mass.
1 mole of any gas at STP = 22.4 L
Molarity.
Limiting factors, theoretical and actual yields.
Gravimetric and volumetric analysis.
Determine empirical and molecular formulas. |
Normality.
% concentration.
Molality.
|
2.03 Identify various types of chemical equations and balance those equations:
2.031 Single replacement.
2.032 Double replacement.
2.033 Decomposition.
2.034 Synthesis.
2.035 Combustion. (7%) |
Use references table on reaction types to identify reaction types and predict products.
Use activity series for single replacement.
Use solubility table and/or solubility rules for double replacement.
Write ionic and net ionic equations.
Arrhenius acid/base neutralization reactions. |
|
2.04 Calculate quantitative relationships in chemical reactions. (stoichiometry)
(7.5%) |
stoichiometry
mole-mole problems
mass-mass problems
mass-volume problems
volume-volume problems
gas laws and PV=nRT
molarity |
|
2.05 Identify the indicators of chemical change:
2.051 Formation of a precipitate.
2.052 Evolution of a gas.
2.053 Color change.
2.054 Absorption or release of heat. (4%) |
Recognize occurrence of reaction based on indicators of change such as formation of precipitate, evolution of a gas, color change and/or energy changes.
Use the solubility rules and activity series in reference materials to predict the outcome of reactions. |
|
2.06 Track the transfer of electrons in oxidation/reduction reactions and assign oxidation numbers:
2.061 Identify the oxidizing and reducing agents
2.062 Assess practical applications of oxidation and reduction reactions.
(4%) |
Using PT and ion chart, assign oxidation state for each element in a compound.
Show transfer of electrons by writing simple half reactions. Only simple metal/metallic ions will be tested.
Determine Voltage calculations.
Identify the element or ion oxidized, element or ion reduced, oxidizing agent, and reducing agent.
Know that redox reactions occur in batteries, combustion, corrosion, and electroplating. |
Redox equation balancing.
Primary vs secondary cells.
|
| COMPETENCY GOAL 3: The learner will build an understanding of energy changes in chemistry. (18% of curriculum) |
3.01 Observe and interpret changes (emission/absorption) in electron energies in the hydrogen atom including the quantized levels and their relationship to atomic spectra:
3.011 Electromagnetic radiation.
3.012 Light.
3.013 Photons. (3%) |
Hydrogen spectrum and Bohr model, electron transfer between "orbits" and relation to energy given off as light. Use reference table.
Use electromagnetic spectrum to relate wavelength and energy. Use equations only as illustration of relationship between energy and wavelength. c= fl, E=hf.
Particle and wave nature of light. |
No calculation of wavelength between two Bohr orbits. |
3.02 Analyze the law of conservation of energy, energy transformation, and various forms of energy involved in chemical reactions.
(5%) |
Connect to 3.04 - calorimetry, calculations of heat based on temperature change of a quantity of water, q=mcDT, definitions of enthalpy, exothermic, endothermic, heats of reaction and stoichiometry.
Energy vs pathway diagram showing energy of reactants, energy of products, enthalpy change, activation energy for exothermic and endothermic reactions.
Heating and cooling curves.
Phases Diagrams |
Hess's law, enthalpy calculations, heats of formation. |
3.03 Compare and contrast the nature of heat and temperature.
(4%) |
Temperature as measure of average kinetic energy of molecules.
Heat as q=mcDT, energy transferred from hot to cold.
Specific heat Specific heat. |
|
3.04 Analyze calorimetric measurement in simple systems and the energy involved in changes in state.
(5%) |
Calorimetry applications.
Heating curve for ice and or water showing plateaus at phase changes, include heat of fusion, vaporization for water. |
|
3.05 Analyze the relationship between energy transfer and disorder in the universe:
3.051 Nuclear.
3.052 Fossil fuels, Solar, Alternative sources.
(2%) |
General knowledge of how a nuclear reactor works.
Describe energy sources and the pros/cons of each energy source.
Definition of entropy and its implications. |
Calculations of entropy, enthalpy or Gibbs free energy. |
| COMPETENCY GOAL 4: The learner will build an understanding of equilibrium and kinetics. (16% of curriculum) |
4.01 Explain the dynamics of physical and chemical equilibria:
4.011 Phase changes.
4.012 Forward and reversible reactions.
(3%) |
Understand ice/water and water/vapor equilibrium.
Understand that some reactions don't go to completion, and an equilibrium is established. Write equilibrium express but no calculations.
Phase diagrams. |
Equilibrium expressions or Calculations.
Triple point.
|
4.02 Explain the factors that alter the equilibrium in a chemical reaction.
(4%) |
Le Chatelier's Principle - concentration, pressure, temperature.
Use the terms "shift to the right, shift to the left" or make more produce, make more reactant to describe changes.
Equilibrium expression as it relates to weak and strong acids but no calculations. |
Equilibrium expressions or calculations. |
4.03 Assess reaction rates and factors that affect reaction rates.
(4%) |
Rate as change in concentration (or pressure) as function of time. Factors affecting rate: concentration, pressure, temperature, catalyst (lower activation energy). |
Reaction order, time/concentration equations. Reaction mechanisms or rate determining steps. |
4.04 Compare and contrast the nature, behavior, concentration, and strength of acids and bases:
4.041 Acid-base neutralization.
4.042 Degree of dissociation or ionization.
4.043 Electrical conductivity.
4.044 pH.
(5%) |
Properties of acids and bases; Strength vs concentration; strength of weak acids and bases - partial dissociation.
Arrhenius and Bronsted.Lowery theories
Acid/base titration and stoichiometry. (nMV = nMV)
Weak Vs strong acids
pH scale and calculations with
pH=-log[H+], pOH=-log[OH-], pH + pOH = 14, [OH]= 10-pOH[H+]=10-pH
Buffer systems. (qualitative discussion only) |
Acid-base equilibria equations.
Lewis theory.
Ka and Kb calculations
|
|
|
|
 |
Last modified
Contact us for more info

|

|
